copper sulfate gravimetric method|copper sulfate method pdf : custom Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as . Join Crunchyroll, the largest anime and manga streaming plat.
{plog:ftitle_list}
Resultado da let's go to the gym! kelsecrets2 (@kelsecrets) on TikTok | 10.1K Likes. 3.6K Followers. Hello! Brazilian in Canada Insta x.kelsecrets.Watch the latest .
Copper sulfate gravimetric method. This method is based on the estimation of specific gravity of blood, assuming that the donor has normal protein levels. Specific gravity of 1.053 corresponds to an Hb level of 12.5 g/dL.NHS Blood and Transplant's (NHSBT) customary method have been capillary gravimetry (copper sulphate), followed by venous spectrophotometry (HemoCue) for donors failing .Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as . Other standard methods for the determination of sulfate in water and wastewater include ion chromatography . A 0.7336-g sample of an alloy that contains copper and zinc is dissolved in 8 M HCl and diluted to 100 mL in a .
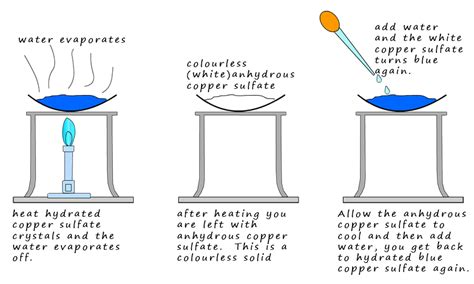
In this experiment students will measure the mass of hydrated copper(II) sulfate before and after heating and use mole calculations to find the formula. . 2g Evaluate methods and suggest possible improvements and further .The Munson and Walker method is an example of a gravimetric method of determining the concentration of reducing sugars in a sample. Carbohydrates are oxidized in the presence of heat and an excess of copper sulfate and alkaline tartrate under carefully controlled conditions which leads to the formation of a copper oxide precipitate:
Using Mass as an Analytical Signal; Types of Gravimetric Methods; Conservation of Mass; Why Gravimetry is Important; Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry.Later, as you read through the descriptions of specific gravimetric methods, this survey will help you focus on their . Qualitative copper sulfate gravimetric method has been the archaic time-tested method that is still used in resource-constrained settings. Portable hemoglobinometers are modern quantitative devices that have been further modified to reagent-free cuvettes. Furthermore, noninvasive spectrophotometry was introduced, mitigating pain to blood donor .
vochtmeter planten tabel
GRAVIMETRIC METHOD Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Gravimetry = analytical methods that measure the mass or mass changes. Volatilization gravimetry describes gravimetric analysis methods that use thermal or chemical energy to separate the substances of a mixture or chemical compound. The thermal or chemical energy is used to convert some solid reactant molecules into gaseous molecules. . The anhydrous copper(II) sulfate crystals can be converted back into blue .12A-1 Properties of Precipitates and Precipitating Reagents A gravimetric precipitating agent should react specifically or at least selectively with the analyte and give precipitates that is: 1. Enough particle size for retaining on filter paper 2. High purity (free of contaminants) 3. Low solubility that no significant loss of the analyte occurs during filtration Gravimetric analysis is a quantitative method of measurement in chemistry. This measurement method is often used in analytical chemistry because of its high rates of precision and accuracy.
30 GLASGOW MEDICAL JOURNAL Method IT. Drops of heparinized blood were let fall into a graded series of solutions of copper sulphate whose specific gravities ranged from 1.046 to 1.066. The solution of copper sulphate in which the drop of blood remained stationary, after breaking the surface film of the solution, was of the same specific gravity as that of the blood under .Sulfate in aqueous solutions may be determined by a gravimetric method in which sulfate is precipitated as barium sulfate; the method is suitable for sulfate concentrations above 10 mg/litre (ISO, 1990). 3. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE 3.1 Air Levels of sulfate in air in Ontario, Canada, have been found to range from 3.0 to 12.6 µg .In an experiment entitled "Gravimetric Analysis" which aims to find out how to work, application, and requirements of the gravimetric method. In addition to being able to analyze the metal content in compounds, for example in iron (III) oxide and copper sulfate pentahydrate. The experiment uses redox reactions as the basic principle. Background In resource poor settings where automated hematology analyzers are not available, the Cyanmethemoglobin method is often used. This method though cheaper, takes more time. In blood donations, the semi-quantitative gravimetric copper sulfate method which is very easy and inexpensive may be used but does not provide an acceptable degree of .
Water sulfate testing is an important step in ensuring the safety and quality of water. There are a variety of methods available for testing water sulfates, each with their own advantages and disadvantages. In this paper, we will delve into the most common and widely used methods for testing water sulfates, such as gravimetric analysis, ion chromatography, and .
This improved experiment yields data accurate enough to demonstrate sequential gravimetric analysis. . This study examined how to produce new methods of copper (II) sulfate crystallization by using a small-scale chemistry tool such as small-scale reaction surface and petri dish. The making of .
The use of the copper sulphate method is based on the idea that when a drop of whole blood is allowed to fall into a solution of copper sulphate, an insoluble copper proteinate is created.Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass. In blood donations, the semi-quantitative gravimetric copper sulfate method which is very easy and inexpensive may be used but does not provide an acceptable degree of accuracy. The HemoCue ® hemoglobin photometer has been used for these purposes. This study was conducted to generate data to support or refute its use as a point-of-care device . Copper sulfate gravimetric method. This method is based on the estimation of specific gravity of blood, assuming that the donor has normal protein levels. Specific gravity of 1.053 corresponds to an Hb level of 12.5 g/dL. A drop of blood, allowed to fall into a copper sulfate solution of specific gravity 1.053, becomes encased in a sac of .
In the former, the thiosulfate ion is oxidized to sulfate as well as to the tetrathionate. In the latter, the thiosulfuric acid formed undergoes an internal oxidation-reduction reaction to sulfurous acid and sulfur. . The basic reaction in the determination of copper using the iodometric method is represented by the equation: \[2Cu^{2+} + 4I .
Using Mass as an Analytical Signal; Types of Gravimetric Methods; Conservation of Mass; Why Gravimetry is Important; Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry.Later, as you read through the descriptions of specific gravimetric methods, this survey will help you focus on their .Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of . Background: The National Blood Transfusion Service (NBTS) in Tanzania uses the Copper Sulphate (CuSO4) gravimetric method to estimate hemoglobin (Hb) in blood donors. However, this and other point-of-care methods, including HemoCue, may provide false results. Therefore, this study aimed to evaluate the performance of CuSO4 and HemoCue methods .
In the case of unknown carbonates, adding calcium chloride to the unknown solution will precipitate calcium carbonate and the reaction method can proceed as above. Another common gravimetric scenario is determining the water of crystallisation of a known sulfate compound, such as magnesium or copper(II) sulfate.
In blood donations, the semi-quantitative gravimetric copper sulfate method which is very easy and inexpensive may be used but does not provide an acceptable degree of accuracy. The HemoCue® hemoglobin photometer has been used for these purposes. This study was conducted to generate data to support or refute its use as a point-of-care device .4) Suppose that a small portion of the sulfate precipitated as lead sulfate rather than as barium sulfate. How this would change the result of the analysis? 5) From the following list, identify the interfering species in the sulfate determination method used in this experiment: Pb2+, Na+, NO 3-, CO 3 2-, PO 4 3-.
qualitative copper sulfate method

WEBPlease open Telegram to view this post. VIEW IN TELEGRAM. 31.0K views edited 11:19
copper sulfate gravimetric method|copper sulfate method pdf